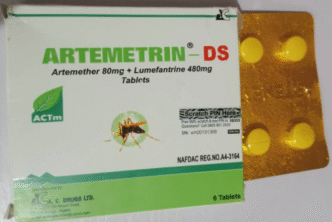
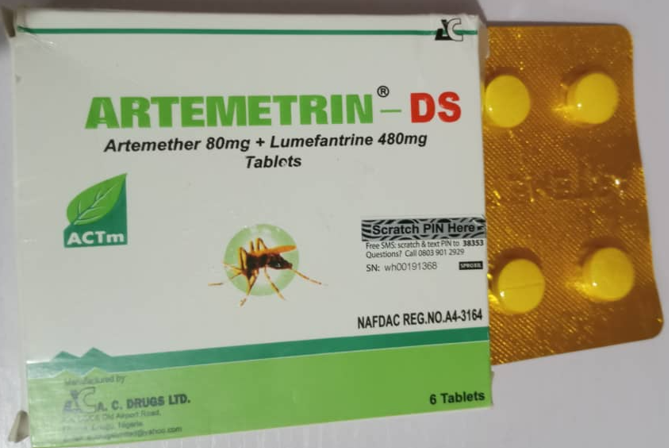
The National Agency for Food and Drug Administration and Control (NAFDAC) has warned the public about falsified versions of two essential medicines — the anti-malarial Artemetrin DS and the antibiotic Ciprofit 500 — citing serious health risks.
Gatekeepers News reports that in a public alert (No. 030/2025), the agency said the counterfeit drugs, though obtained from licensed vendors and wholesalers, were found to contain dangerously low levels of active ingredients.
Laboratory tests revealed that Artemetrin DS, labelled as produced by A.C. Drugs Ltd in Enugu State, contained only 59.2 per cent artemether and 71.2 per cent lumefantrine, far below the acceptable 90–110 per cent potency range.
Similarly, Ciprofit 500, falsely branded as manufactured by Impact Pharmaceutical Ltd, was discovered to have just 5.7 per cent ciprofloxacin. Neither product is registered in NAFDAC’s official database, and both carried fabricated registration numbers.
“The implications of substandard anti-malarials in a malaria-endemic country like Nigeria cannot be overstated. These falsified products undermine treatment efficacy and could lead to preventable deaths,” the agency warned.
NAFDAC urged Nigerians to stop using the medicines, return any stocks to its offices, and seek immediate medical attention if they had experienced treatment failure or adverse reactions.
The agency noted that the World Health Organisation estimates 10 per cent of medicines globally are falsified, with Nigeria facing a potentially higher prevalence. This poses a major setback in tackling malaria, which kills over 200,000 Nigerians annually, and drug-resistant bacterial infections.
The warning follows the seizure of fake malaria drugs worth ₦1.2 billion in Lagos on September 12. Earlier in 2025, the agency also flagged falsified contraceptives, oxytocin injections, and milk products.
NAFDAC advised healthcare workers, distributors, and consumers to verify all medicines through its online portal, purchase only from authorised outlets, and report suspicious products via its hotline or email.
Director-General Prof. Mojisola Adeyeye reaffirmed the agency’s commitment to curbing counterfeit medicines.
“With the full support of the government, we will continue to intensify surveillance and enforcement to eliminate these threats,” she said.